Oseltamivir, widely recognized by its brand name Tamiflu, is a crucial antiviral medication primarily used for the prevention and treatment of influenza A and B viruses. Its development marked a significant advancement in combating seasonal flu and pandemic influenza threats. Understanding its mechanism of action, applications, and impact is vital for public health strategies and individual preparedness. This article delves into what oseltamivir is for, exploring its role in managing influenza.
Understanding Influenza and the Need for Antivirals
Influenza, commonly known as the flu, is a highly contagious respiratory illness caused by influenza viruses. These viruses are constantly evolving, leading to seasonal epidemics and the potential for more severe pandemics. The Centers for Disease Control and Prevention (CDC) estimates that in the United States, during the 2022-2023 season, there were at least 26 million illnesses, 360,000 hospitalizations, and 24,000 deaths due to influenza. This underscores the significant public health burden and the importance of effective countermeasures.

The Influenza Virus and Its Lifecycle
Influenza viruses are RNA viruses belonging to the Orthomyxoviridae family. They are characterized by their surface glycoproteins, hemagglutinin (HA) and neuraminidase (NA), which are crucial for viral entry into host cells and the release of new virions, respectively. The virus primarily infects the epithelial cells of the respiratory tract.
The influenza virus lifecycle begins with the attachment of the virus to sialic acid receptors on the host cell surface via its hemagglutinin protein. Following entry into the cell, the viral RNA is released into the cytoplasm, where it is replicated and transcribed in the nucleus. New viral proteins are synthesized, and these assemble with viral RNA to form new virions. A critical step in the release of newly formed virions from the infected host cell is the cleavage of sialic acid receptors by the viral neuraminidase enzyme. Without this enzymatic activity, the newly formed viruses would remain tethered to the host cell, preventing their spread to infect other cells.
Why Antivirals are Essential
While vaccines are the cornerstone of influenza prevention, antiviral medications play a vital role in managing the illness. They are particularly important for individuals at high risk of developing serious complications from the flu, such as the elderly, young children, pregnant women, and individuals with certain chronic medical conditions. Antivirals can reduce the severity of illness, shorten the duration of symptoms, and prevent serious complications, including pneumonia, bronchitis, sinus infections, and ear infections. In some cases, influenza can lead to hospitalization and even death. Antivirals can also be used prophylactically to prevent infection in individuals who have been exposed to the virus and are at high risk.
Oseltamivir: Mechanism of Action and Development
Oseltamivir’s effectiveness stems from its targeted action against a specific enzyme essential for the influenza virus’s lifecycle. This targeted approach differentiates it from other medications and highlights the sophistication of modern antiviral therapy.
Targeting Neuraminidase: The Key to Oseltamivir’s Efficacy
Oseltamivir is a neuraminidase inhibitor. Neuraminidase is a critical enzyme found on the surface of influenza viruses. Its primary function is to cleave sialic acid residues from the surface of host cells and from newly formed viral particles. This cleavage is essential for two key processes:
- Release of new virions: After new virus particles are assembled within an infected cell, they bud off from the host cell membrane. If neuraminidase were not present to break the bonds between the viral hemagglutinin and the sialic acid on the host cell, the new viruses would remain attached and unable to spread to infect other cells.
- Preventing aggregation of viral particles: Neuraminidase also prevents newly released viruses from aggregating with each other, which would hinder their ability to spread.
By inhibiting the activity of neuraminidase, oseltamivir prevents the release of new influenza viruses from infected cells. This action reduces the viral load in the respiratory tract, thereby limiting the spread of the infection within the body and potentially reducing the duration and severity of illness. Oseltamivir is a prodrug, meaning it is inactive in its administered form and is converted into its active metabolite, oseltamivir carboxylate, in the liver. This active metabolite is responsible for inhibiting the neuraminidase enzyme.
The Discovery and Evolution of Neuraminidase Inhibitors
The development of neuraminidase inhibitors like oseltamivir was a significant breakthrough in influenza treatment. Prior to their availability, treatment options for influenza were limited, primarily focusing on supportive care. The understanding of influenza virus structure and function, particularly the roles of hemagglutinin and neuraminidase, paved the way for the design of drugs that could specifically target these viral components.
The first neuraminidase inhibitors were developed in the 1990s. Zanamivir (Relenza) was the first neuraminidase inhibitor approved by the FDA in 1999. Oseltamivir (Tamiflu) followed shortly after, receiving FDA approval in 2000. These drugs represented a new class of antivirals with a specific mechanism of action, offering a proactive approach to managing influenza beyond just symptomatic relief. Subsequent research has continued to explore and develop new antiviral agents, including those that target different stages of the viral lifecycle or possess broader antiviral activity.
Applications of Oseltamivir: Treatment and Prevention
Oseltamivir serves two primary purposes in managing influenza: treating active infections and preventing the onset of illness in exposed individuals. Its effectiveness in both scenarios makes it a valuable tool for public health.
Treating Influenza Infections
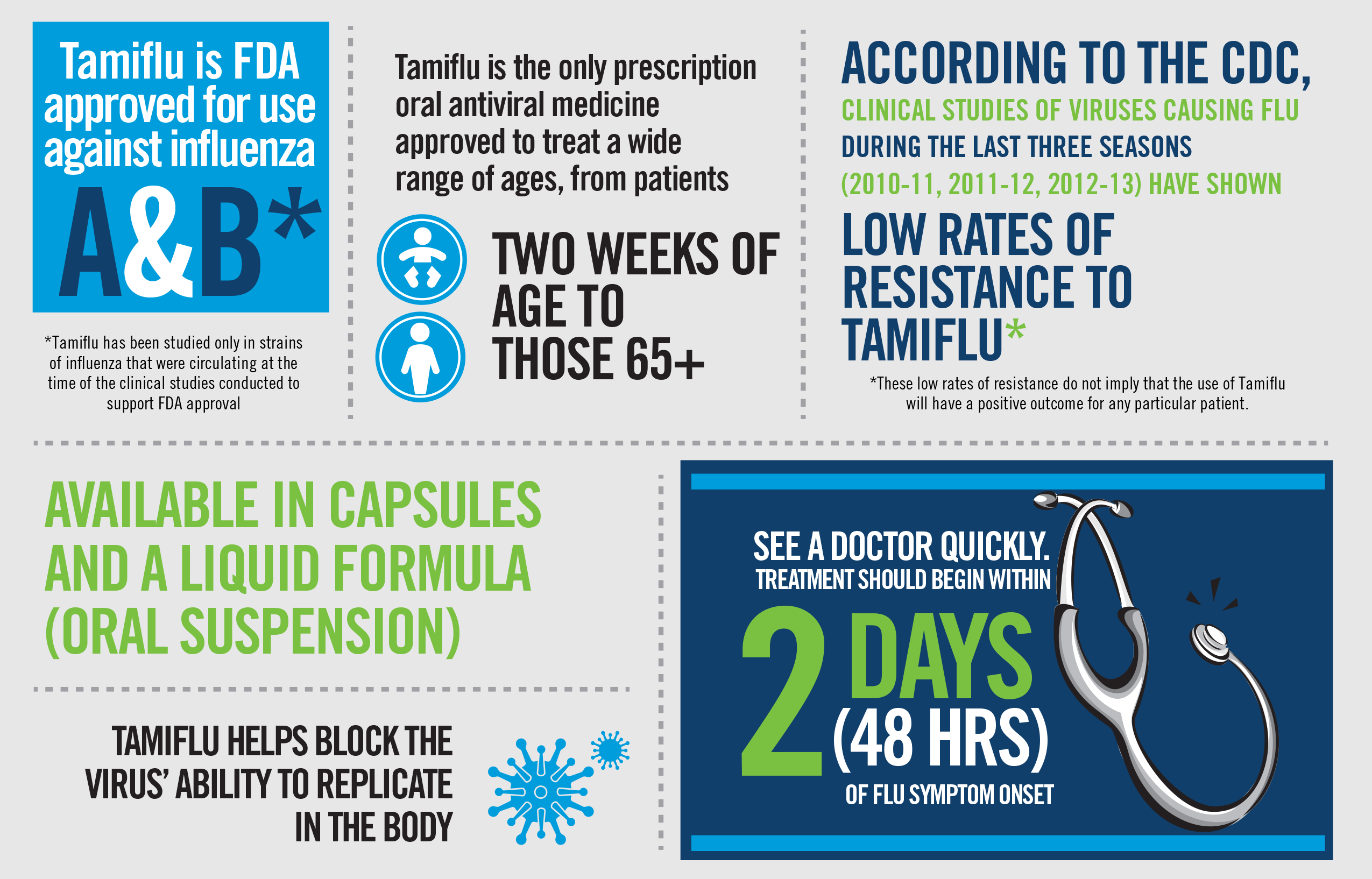
Oseltamivir is prescribed to individuals diagnosed with influenza, particularly those who are experiencing symptoms or are at increased risk of developing complications. The drug is most effective when initiated early in the course of illness, ideally within 48 hours of symptom onset. Starting oseltamivir promptly can lead to a reduction in the duration of flu symptoms, such as fever, cough, sore throat, and body aches. It can also decrease the risk of flu-related complications.
The standard treatment regimen for influenza in adults typically involves taking 75 mg of oseltamivir twice daily for five days. Dosing may be adjusted for individuals with impaired kidney function. For children, the dosage is based on their weight. It is crucial to complete the full course of medication as prescribed by a healthcare professional, even if symptoms begin to improve, to ensure the virus is fully suppressed and to prevent the development of drug resistance.
Prophylaxis: Preventing Influenza Transmission
Beyond treating active infections, oseltamivir is also used for post-exposure prophylaxis. This means it can be given to individuals who have been in close contact with someone who has confirmed influenza but have not yet developed symptoms. The goal of prophylaxis is to prevent the individual from becoming infected or to reduce the severity of illness if infection does occur.
Oseltamivir prophylaxis is often recommended for individuals at high risk of severe complications from influenza who have been exposed to the virus. This can include household contacts of a confirmed case or individuals in settings where transmission is likely, such as long-term care facilities. The typical dosage for prophylaxis is 75 mg once daily for at least 10 days, starting as soon as possible after exposure. The decision to use oseltamivir for prophylaxis is made on a case-by-case basis by a healthcare provider, considering the individual’s risk factors, the potential for exposure, and the overall prevalence of influenza in the community.
Considerations and Limitations of Oseltamivir
While oseltamivir is a powerful tool against influenza, it is not without its considerations and limitations. Understanding these aspects is crucial for its responsible use and for developing comprehensive influenza management strategies.
When Oseltamivir Might Not Be Prescribed
Several factors influence the decision to prescribe oseltamivir. It is primarily indicated for confirmed or suspected influenza. If symptoms are mild and the individual is otherwise healthy, a healthcare provider might opt for supportive care, focusing on rest, hydration, and over-the-counter medications for symptom relief, as the risks and benefits of antivirals may not strongly favor their use.
Furthermore, oseltamivir is most effective when taken early in the illness. If an individual presents with flu-like symptoms that have been present for more than 48 hours, the benefit of starting oseltamivir may be significantly reduced, although it may still be considered for high-risk individuals. The development of antiviral resistance by influenza viruses is another concern. While resistance to oseltamivir has been observed, it is not widespread for all circulating strains, and resistance patterns can change. Healthcare providers monitor these trends to guide treatment decisions. Finally, oseltamivir may not be suitable for individuals with certain severe allergies to its components or specific medical conditions that contraindicate its use.
Drug Resistance and Future Directions
The emergence of drug resistance is a perpetual challenge in the field of antiviral therapy. Influenza viruses have the capacity to mutate, and over time, these mutations can lead to strains that are less susceptible or completely resistant to existing antiviral medications. The neuraminidase enzyme, like other viral proteins, can undergo changes that reduce the binding affinity of neuraminidase inhibitors like oseltamivir, rendering them less effective.
The World Health Organization (WHO) and national public health agencies continuously monitor influenza virus strains for resistance patterns. This surveillance is critical for informing treatment guidelines and vaccine development. When resistance emerges, it highlights the ongoing need for research and development of new antiviral drugs with novel mechanisms of action. Future directions in influenza antiviral therapy may include developing drugs that target different viral proteins or different stages of the viral replication cycle, or exploring combination therapies that reduce the likelihood of resistance developing. The ongoing evolution of influenza viruses necessitates a proactive and adaptive approach to antiviral development and deployment.
